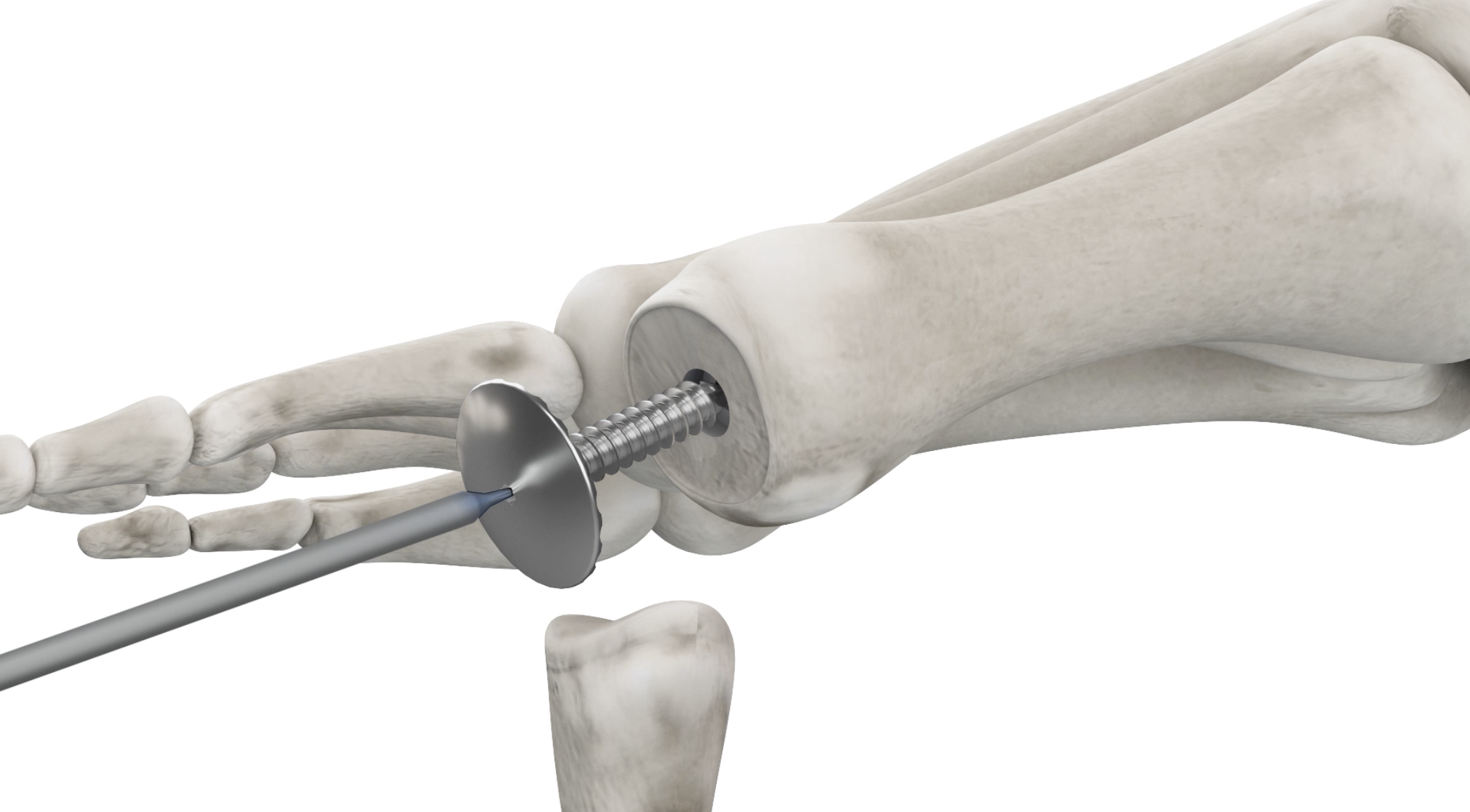
Choose function over fusion
The Accu-Joint® implant system is a revolutionary, FDA-approved treatment for MTP joint disorders that is designed to restore natural motion and preserve your active lifestyle.
You Have a Choice. Preserve Your Joint
The Accu-Joint® Implant is the only implant of its kind. Unlike other implants that remove large portions of the bone and transfer weight onto the implant itself, the Accu-Joint® preserves bone and is non-weight-bearing. This translates to a stronger joint, reduced pain, and increased mobility.
- No resection of the bone end: Patented instruments preserve the bone by resurfacing the bone end without the need for resection.
- Non-weight bearing design: Body weight is transferred to your existing anatomy instead of the implant.
- Fast, active recovery: No bone resection means you have a fast recovery and can put weight on your toe from day one.
- Keep your options open: With retained bone you can pursue other treatment options in the future if necessary.
“We're not removing a lot of bone”
“My patients with the Accu-Joint® implant are the happiest of all my patients. They're walking in to follow up appointments, they're moving the foot early, the swelling is minimal, and there's really not a lot of pain. The Accu-Joint® doesn’t burn any bridges. We're not removing a lot of bone, so if we need to ever do anything again in the future, all the options exist.”
—Dr. Stephen Arndt, MD
The Accu-Joint® vs Resection
Preserve more of your natural anatomy with resurfacing, which focuses on cartilage removal and only 1 mm of bone rather than removing 5mm or more of the joint bone. By retaining bone structure, resurfacing provides more stability and flexibility.
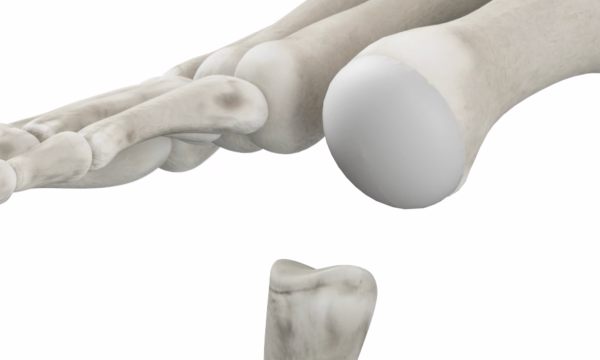
Other Implants
Resect the Joint
- Bone saw is used to cut off joint bone end
- Implant replaces entire joint
- Body weight is on the implant which can loosen and/or fail
- Loss of bone limits your future options and revision surgery is costly and painful with a lengthy recovery
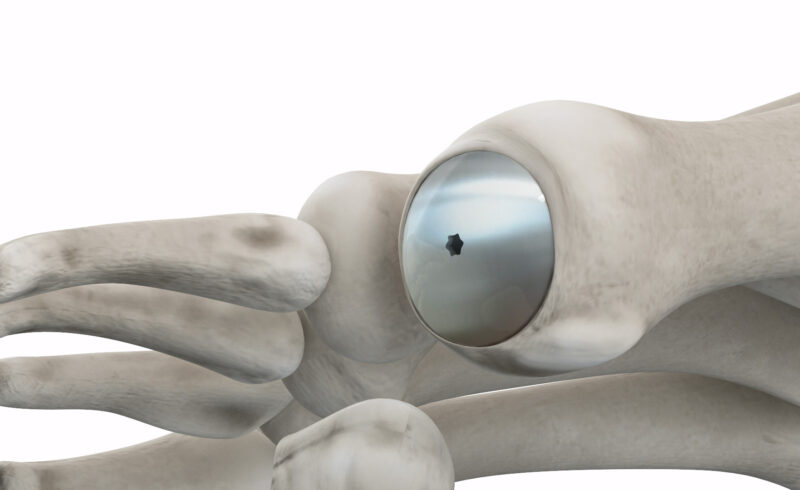
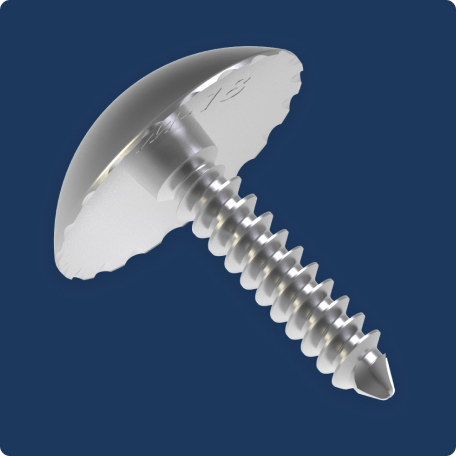
Accu-Joint® Implant
Preserve the Joint
- Patented tool removes only cartilage, minimal bone loss
- Implant replaces cartilage, not the joint
- Body weight is on your existing anatomy, not the implant
- Bone retention means you can pursue other options if necessary
The Accu-Joint® vs Fusion
Joint fusion is currently the most prevalent way to treat MTP joint disorders. But joint fusion is an irreversible procedure that should only be used as a last resort. While joint fusion does reduce MTP joint pain, it also restricts (LOCKS) joint movement and makes any future procedures painful and difficult.
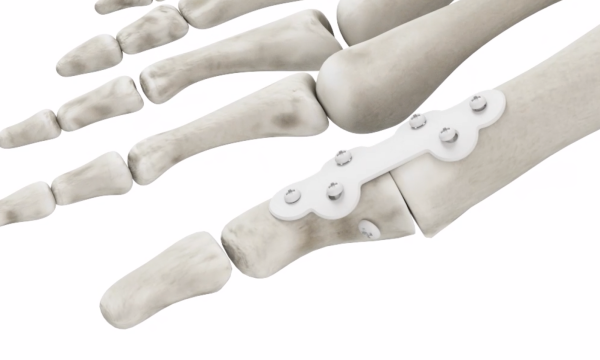
Joint Fusion
- Bone saw is used to cut off joint bone end
- Plates and screws connect the sides of the joint together
- The toe joint cannot bend, limiting your activity level
- Toe length is generally reduced
- Revision surgery is costly and painful with a lengthy recovery and an uncertain outcome.
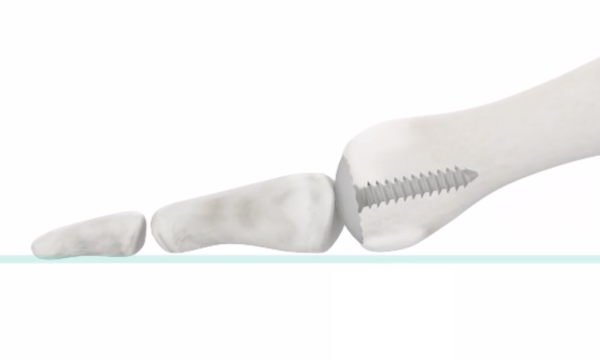
The Accu-Joint® Implant
- Restores joint motion – replaces cartilage worn away from arthritis
- Preserves anatomy which exists to bear body weight
- Less removal of bone means your weight bearing on day one
- Length of the toe doesn’t change
- Bone retention means you can pursue other options if necessary
“My toe pain disappeared”
Over the years I’ve had four separate surgeries to try and relieve the joint pain in my left big toe. None of them worked. With each surgery my pain got worse. After the Accu-Joint® Implant surgery, my toe pain disappeared. Not only that, I now have range of motion in my joint and can walk normally. The results are a miracle.
— Aly, Accu-Joint® Implant Patient
Unlike bone fusions, which restrict movement, the Accu-Joint® Implant gives your toe the ability to flex!
When you choose function over fusion, amazing things are possible!
Hear from more patients about how the Accu-Joint® implant reduced their pain and changed their lives.
Watch Patient Stories
Who is the Accu-Joint® Implant for?
The Accu-Joint® implant should be used to treat patients with degenerative and post-traumatic arthritis of the MTP joint who have hallux limitus or hallux rigidus, or any arthritic conditions having unstable or painful MTP joints. The Implant is intended to be used with bone cement.*
*At the surgeon’s discretion/decision

Choose function over fusion with the Accu-Joint® Implant
Learn why the Accu-Joint® implant is the only choice for MTP joint disorders.