FOR SURGEONS
We Reinvented the Hemi
The Accu-Joint® Hemi Implant is the next generation solution that addresses the failures of the past and sets new standards in the treatment of hallux limitus.
- Non-weight bearing
- Doesn’t burn any bridges
- Minimal bone resection
“We're not removing a lot of bone”
“My patients with the Accu-Joint® are the happiest of all my patients. They're walking in to follow up appointments, they're moving the foot early, the swelling is minimal, and there's really not a lot of pain. The Accu-Joint® doesn’t burn any bridges. We're not removing a lot of bone, so if we need to ever do anything again in the future, all the options exist.”
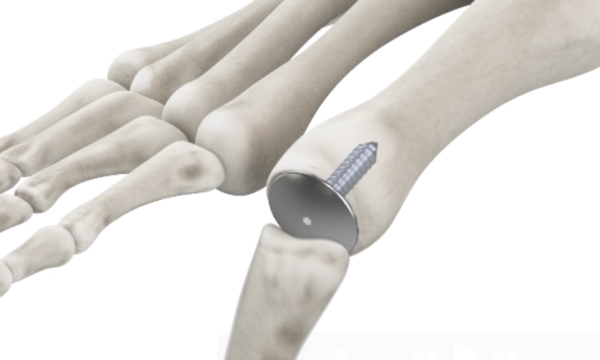
Introducing the Accu-Joint® Hemi Implant
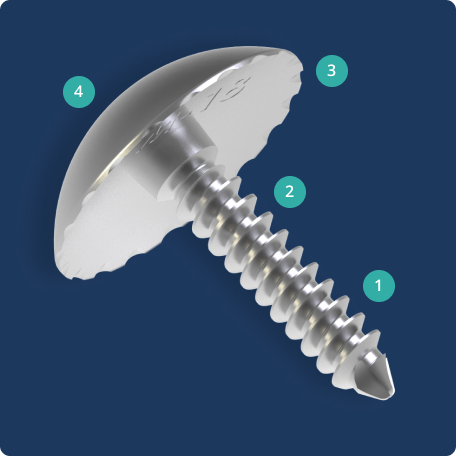
The Accu-Joint® Implant replaces worn cartilage while preserving the subchondral bone end, providing a true articular surface replacement. The implant is sized 10-15% smaller than the bone end diameter so the patient’s existing anatomy, not the implant, bears body weight. By preserving the joint’s anatomical structures, the procedure can help maintain strength and stability for foot function.
- The solid stem with cancellous threads ensures the implant is securely fixed to the hard subchondral bone.
- The threaded barrel, wider than the stem, helps transfer stress from the stem to subchondral bone.
- Grooves and scalloped edges under the head allow for bone ingrowth to help prevent loosening.
- The implant is sized 10% smaller than the bone end to help transfer weight to preserved bone instead of the implant.
A Smarter Way to Treat Joint Pain
The Accu-Joint® Implant is designed to replace worn cartilage without removing large portions of bone. By preserving the patient’s natural anatomy and transferring weight through existing bone structures, the implant can help reduce pain while keeping options open for future treatments.
Smarter Design
- Non-weight bearing
- Keeps options open (Doesn’t burn bridges)
- Rigid fixation
A Better Alternative to Fusion
- Maintain normal gait cycle
- Flexibility with shoe gear
- Fast, active recovery
Anatomic Preservation
- Preserve hard bone for adequate fixation
- Maintains subconderal support structures
- Only 1mm of bone resection
Surgical Technique
Step 1
Obtain full exposure. Perform an aggressive extra articular cheilectomy including the dorsal exostectomy and reduce the joint size to normal.
Step 2
A McGlamry elevator is utilized to free up the sesamoid apparatus and reduce flexor binding. Release of these adhesions is necessary for restoration of anatomic motion. Complete a full 360 degree extra-articular cheilectomy. Resection of the dorsal exostosis is important to reduce the enlarged joint to its original size, and to recreate the dorsal articulation.
Step 3
Center and align the sizer to the bone articular surface. The sizer for the Accu-Joint® Hemi Implant should be positioned perpendicular to the articular set angle with the stem of the implant angled slightly plantarly.
Step 4
The sizer can be used as a guide for the insertion of the K wire and visualizing with C-ARM is essential to ensure the wire is properly placed. 30-40 mm of the wire should be left exposed to aid with instrumentation. The K-wire should be positioned so the stem of the implant rests in the plantar half of the first metatarsal.
Step 5
Use the two-stage reamer gently and lightly with high-speed revolution for removal of worn cartilage and any indicated decompression. The subchondral bone remains preserved for rigid fixation of the Accu-Joint®.
Step 6
Drill the pilot hole until the top of the counterbore is flush with the surface of the bone. For soft bone only drill to the bottom of the counterbore.
Step 7
The trial is placed, the K-wire removed and range of motion simulated. The goal is to achieve 90 degrees of dorsiflexion during this step. If desired range of motion is not achieved, remove the trial and decompress further. Ensure the implant is sized appropriately and does not overhang the prepared surface. The Tap is utilized for the First MPJ to prepare the pilot hole for the implant.
Step 8
Thread the Accu-Joint® Hemi Implant into the pilot hole using the T-6 driver. Ensure 360 degree seating of the implant onto the bone with no gapping. Confirm adequate seating with X-ray.
Step 9
Ensure that smooth range of motion is verified using simulated weight bearing. Flush and closure is completed followed by another final check of the range of motion. Postoperatively, immediate and full weight bearing is allowed using a surgical shoe. During the first 3 days rest, ice, and elevation are encouraged. Further care will follow a normal postoperative protocol.
“My toe pain disappeared”
Over the years I’ve had four separate surgeries to try and relieve the joint pain in my left big toe. None of them worked. With each surgery my pain got worse. After the Accu-Joint® Implant surgery, my toe pain disappeared. Not only that, I now have range of motion in my joint and can walk normally. The results are a miracle.
— Aly, Accu-Joint® Implant Patient
More Patient StoriesUnlike bone fusions, which restrict movement, the Accu-Joint® Implant gives your toe the ability to flex!
Who is the Accu-Joint® Implant for?
The Accu-Joint® Hemi Implant should be used to treat patients with degenerative and post-traumatic arthritis of the MTP joint who have hallux limitus or hallux rigidus, or any arthritic conditions having unstable or painful MTP joints. The Implant is intended to be used with bone cement.*
*At the surgeon’s discretion/decision

Become an Accu-Joint® Implant Surgeon
Join us in the fight for function over fusion. Fill out the form and our team will be in touch to schedule a call.
"*" indicates required fields
This material is intended for health care professionals. Distribution to any other recipient is prohibited. For product information, including indications, contraindications,warnings, precautions, potential adverse effects, and patient counseling information, see the package insert and www.accufixsurgical.com. The Accu-Joint® hemi implant, a hemi-arthroplasty metatarsal head or phalangeal base implant for the metatarsophalangeal (MTP) joint, is indicated for use in the treatment of patients with degenerative and post-traumatic arthritis in the MTP joint in the presence of good bone stock, along with the following clinical conditions: Hallux Limitus, Hallux Valgus, Hallux Rigidus, and an unstable or painful MTP joint. The Accu-Joint® hemi implant is intended to be used with bone cement. The metatarsal head and phalangeal base may not be used together at the same joint. Potential risks include, but are not limited to, risks of the procedure, including the risk of implant breakage, failure or loosening, bone fracture, allergic reaction to implant materials or infection, any of which can require additional surgery. For complete prescribing information, see the package insert and www.accufixsurgical.com.



